A Hydrogen Fuel Cell Produces Which of the Following
In addition to this pure hydrogen type there are hydrocarbon fuels for fuel cells including diesel methanol see. Hydrogen fuel cell vehicles produce one of the following as exhaust AH2O2 BH2O CCH4 DNH3 Answer Explanation Option.

A Basic Overview Of Fuel Cell Technology
Which of the following is the most popular application of hydrogen fuel cell.

. Direct Solar Water Splitting Process. The energy can be supplied flexibly from the fuel cell and battery. Hydrogen fuel cell vehicles produce one of the following as exhaust.
Hydrogen-based fuel cells are more energy-efficient than the traditional combustion engine. The Fuel cells emit only heat and water as a by-product. 5 hours agoCHARLOTTE NC April 26 2022 GLOBE NEWSWIRE -- Terrestrial Energy USA a leading developer of Generation IV fission technology has joined the Fuel Cell and Hydrogen Energy Association FCHEA.
Hydrogen and fuel cells can play an important role in our national energy strategy with the potential for use in a broad range of applications across virtually all sectorstransportation commercial industrial residential and portable. - The oxidation of hydrogen produces a greenhouse gas Some methods of hydrogen production cause pollution - It can be dangerous to store and transport hydrogen Catalysts for fuels cells are often made of rare and expensive metals. Chemical reactions that split water produce hydrogen.
Very small quantities of Greenhouse gases are produced. The main benefit of a battery high voltage battery in fuel cell buses is the increase in efficiency through more efficient energy management. There are wide availability of resources to produce hydrogen.
As of 2020 the majority of hydrogen 95 is produced from fossil fuels by steam reforming or partial oxidation of methane and coal gasification with only a small quantity by other routes such as biomass gasification or electrolysis of water. Hydrogen is a secondary energy source that can be formed from diverse renewable resources like biomass wind solar natural gas and nuclear power. Hydrogen fuel can be produced from methane or by electrolysis of water.
UPSC 2010 CS-P NH3. A combustion engine produces--CO CO2 H2O and energy. Handling of Hydrogen requires utmost care as it is more explosive than petrol.
1H2 is the fuel in--a hydrogen fuel cell 2. D CH4. D H 2 O 2.
Hydrogen is a clean fuel that when consumed in a fuel cell produces only water electricity and heat. A N H 3. There is a wide range of uses for fuel cells because they can run on a wide.
Hydrogen fuel cell vehicles produce one of the following asexhaust. High-Temperature Thermochemical Water Splitting. Uses light energy to split water into hydrogen and oxygen.
The fuel cell reaction is less efficient than the combustion reaction. C NH3. Hydrogen fuel cell vehicles HFCVs have a substantial potential to moderate emissions because they do not produce any greenhouse gases GHGs during vehicle operation.
The Fuel cells emit only heat and water as a by-product. Part of solved Physics questions and answers. The chemical energy of hydrogen or other fuels is harnessed by a fuel cell to produce electricity in an environmentally friendly and cost-effective manner.
Thermochemical processes use heat and chemical reactions to release hydrogen from organic materials such as fossil fuels and biomass or from materials like water. The net reaction to this is exothermic. When consumed in a fuel cell hydrogen generates only water.
Hydrocarbon fuels are used in--a combustion engine 3. Hydrogen fuel cell vehicles produce one of the following as exhaust. Hydrogen can be produced using a number of different processes.
In addition to the main benefit of increased efficiency however there are other reasons. Very small quantities of Greenhouse gases are produced. The amount of emissions associated with producing hydrogen fuels depends on the source of hydrogen and production method.
Direct-methanol fuel cells and indirect methanol fuel cells and chemical hydrides. On the Psychrometric chart the coordinates of any point on it will have abscissa as specific humidity value and ordinate as a dry bulb temperature value. Microorganisms such as bacteria and algae.
C H 2 O. C H 2 O. Handling of Hydrogen requires utmost care as it is more explosive than petrol.
Hydrogen-based fuel cells are more energy-efficient than the traditional combustion engine. These attributes make it an effective alternative fuel option for electricity production applications and transportation. 3 H 2 O.
The waste products with these types of fuel are carbon dioxide and water. B C H 4. When hydrogen is used as a fuel only electricity water and heat are produced.
B H2 O. Currently the majority of hydrogen that is made for use as a fuel comes from natural gas but hydrogen fuel also can be made from water oil coal and plant material. Water H 2 O can also be split into hydrogen H 2 and oxygen O 2 using electrolysis or solar energy.
The combination of the two half cell potentials for the electrochemical reaction creates a positive potential for cells. B 10 Important Books for UPSC Preparation 10 Important. Hydrogen fuel cell vehicles produce one ofthe following as exhaust.
There are wide availability of resources to produce hydrogen. Hydrogen can even be produced from your trash. A hydrogen fuel cell is an electrochemical cell that produces current that can work using a spontaneous redox reaction.
4 H 2 O 2. Uses electricity to split water into hydrogen and oxygen. Hydrogen fuel cell vehicles produce one of the following as exhaust a NH 3 b CH 4 c H 2 O d H 2 O 2 physical chemistry csat 1 Answer 0 votes answered Jun 2 2018 by santoshjha 143k points selected Jun 4 2018 by Vikash Kumar Best answer.
A hydrogen fuel cell produces--H2O and electricity. General Science Physics. A H2 O2.
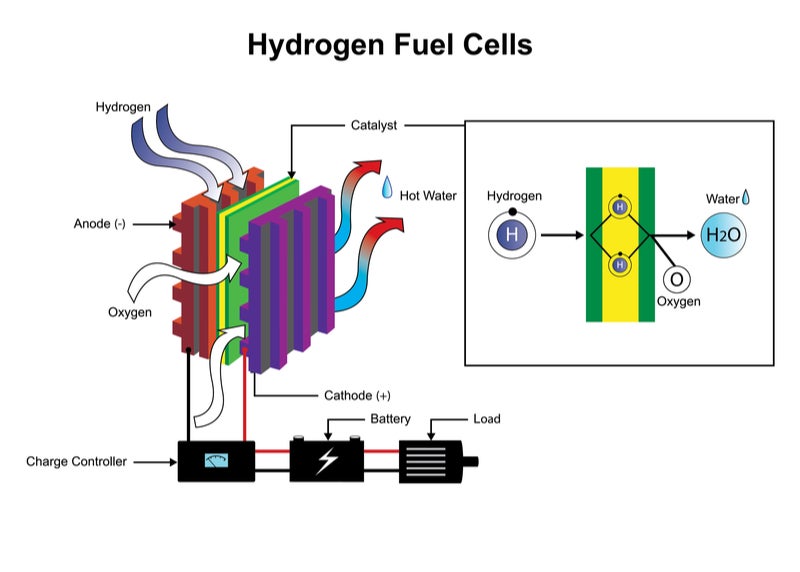
Hydrogen Fuel Cell Overview Of Where We Re At In Hydrocarbon Replacement
No comments for "A Hydrogen Fuel Cell Produces Which of the Following"
Post a Comment